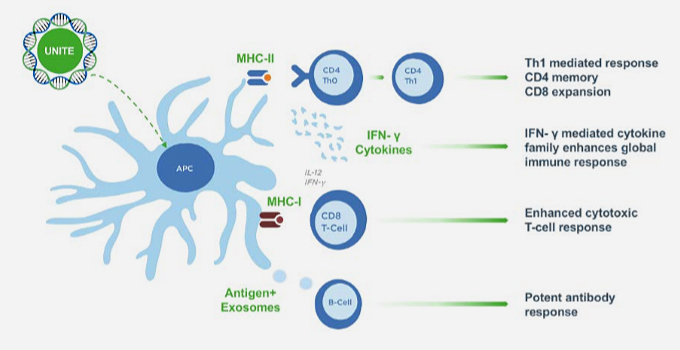
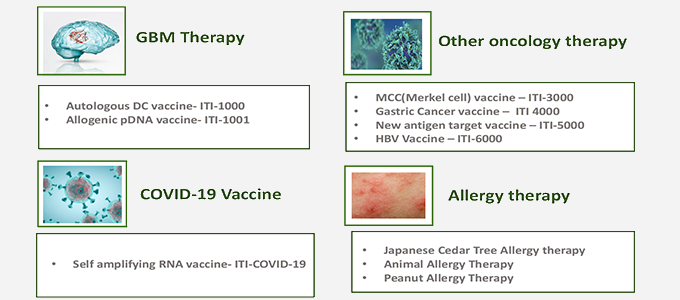
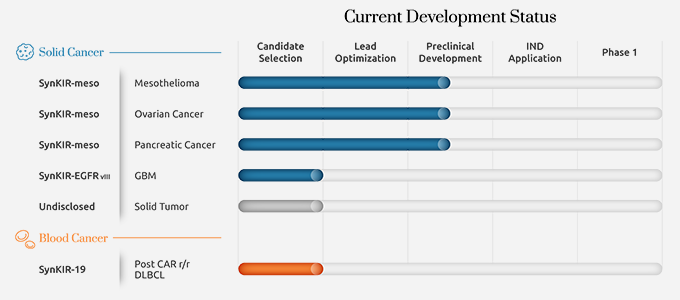
Business Field
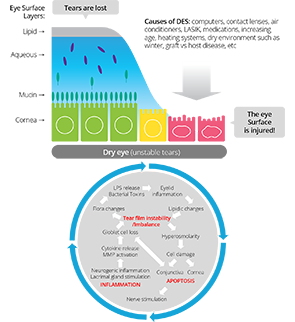
Dry Eye Syndrome (DES) treatment
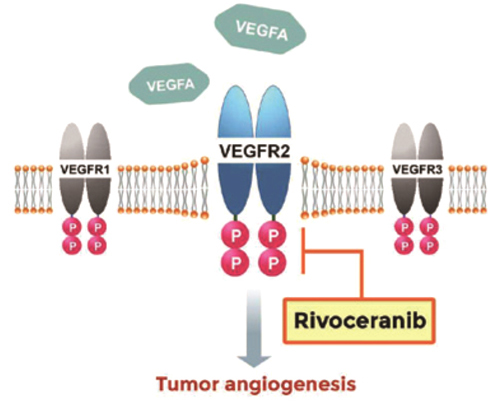
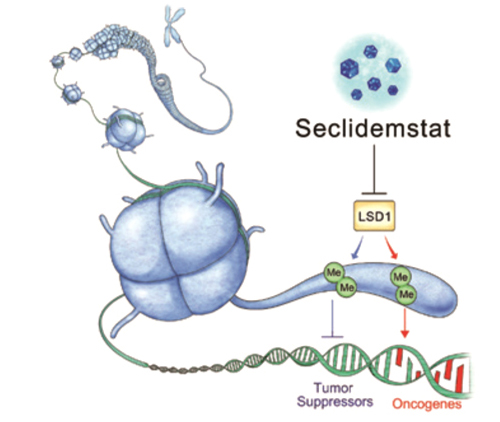
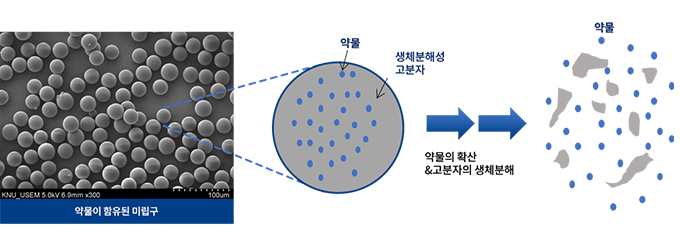
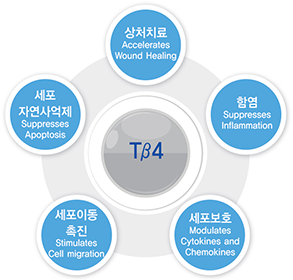
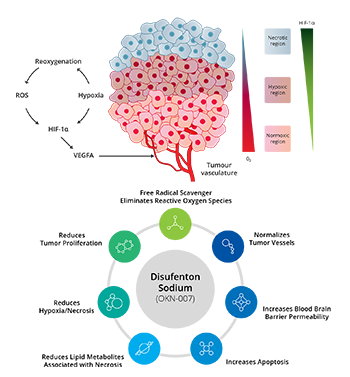
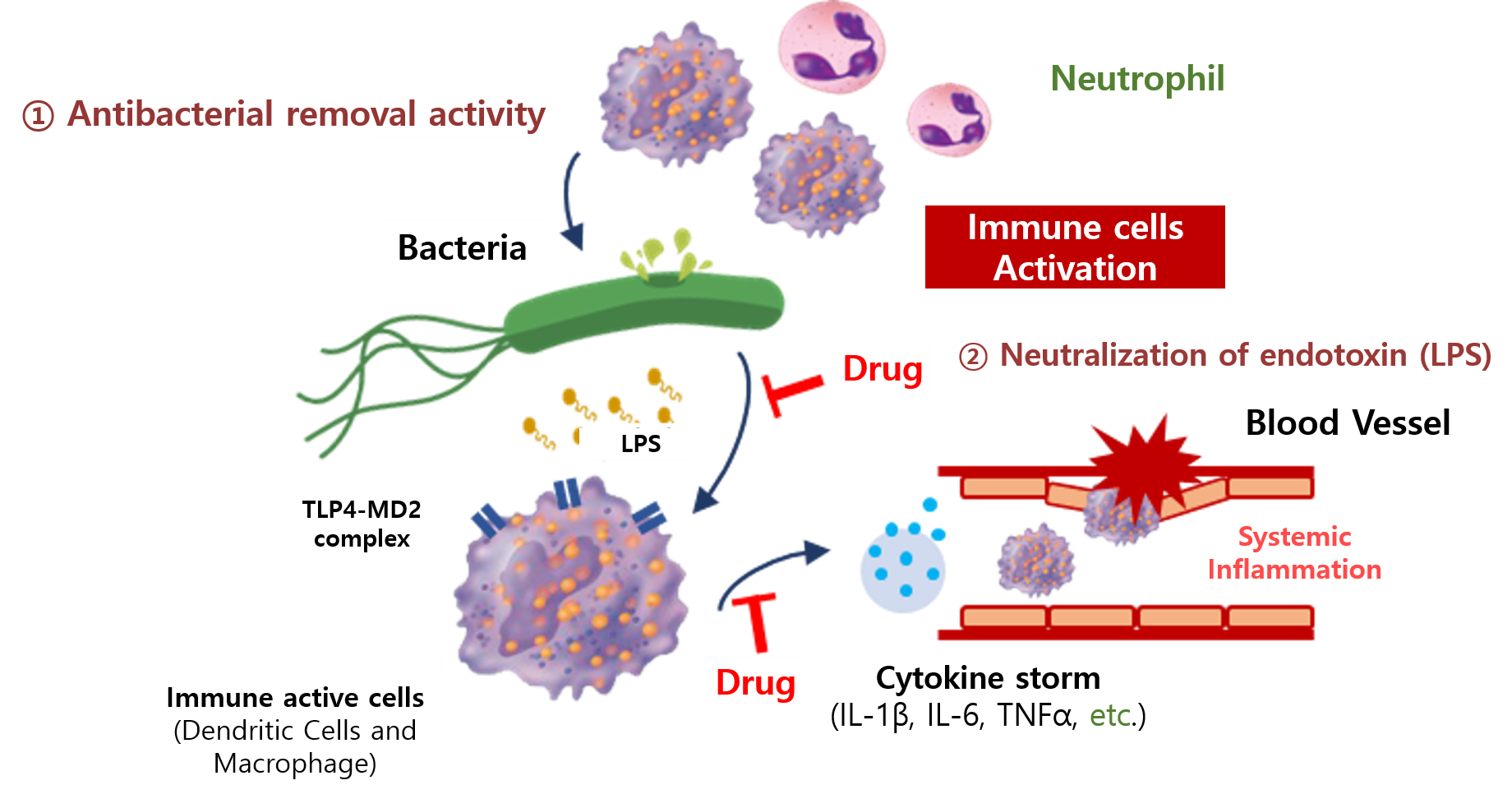
RGN-259 is an anti-inflammatory peptide that is activated in several stages of wound healing and tissue recovery as a medicinal ingredient in Thymosin beta 4. It is a multifunctional substance suitable for dry eye, a multi-personal disease such as tear deficiency or tear membrane disorder caused by excessive tear evaporation and is being developed by a U.S.-based joint venture (ReGen Tree, LLC).
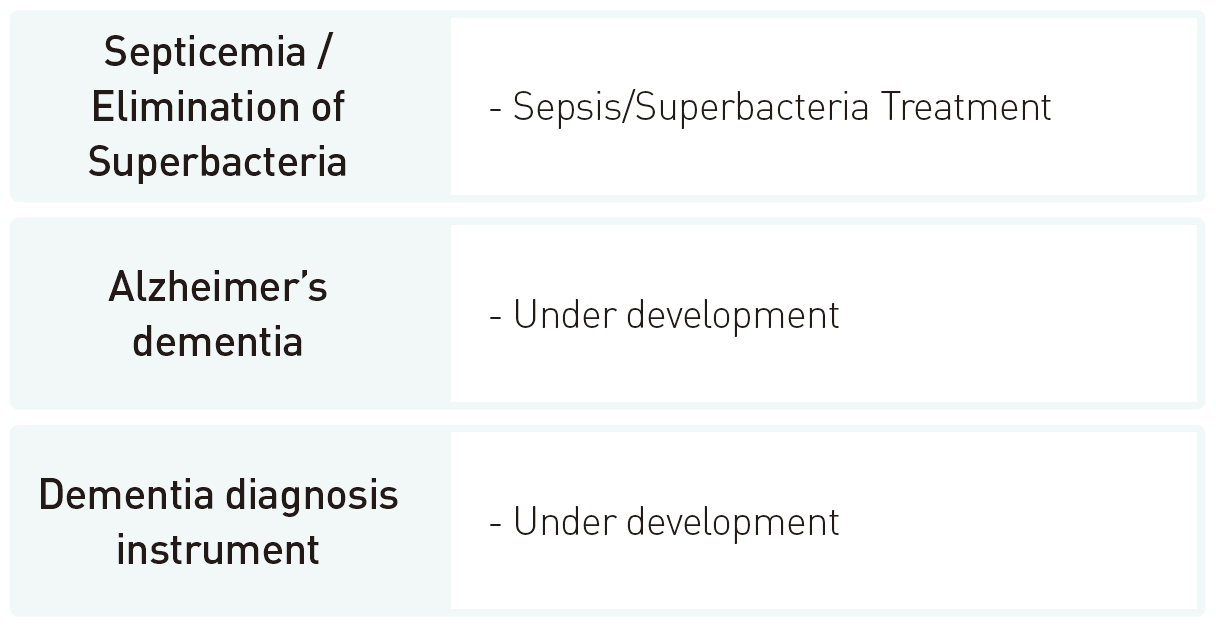
R&D




Product





Product
(30068) 10-5, Myeonghaksandanseo-ro, Yeondong-myeon, Sejong
Tel+82-44-862-9134
Fax+82-44-862-9135